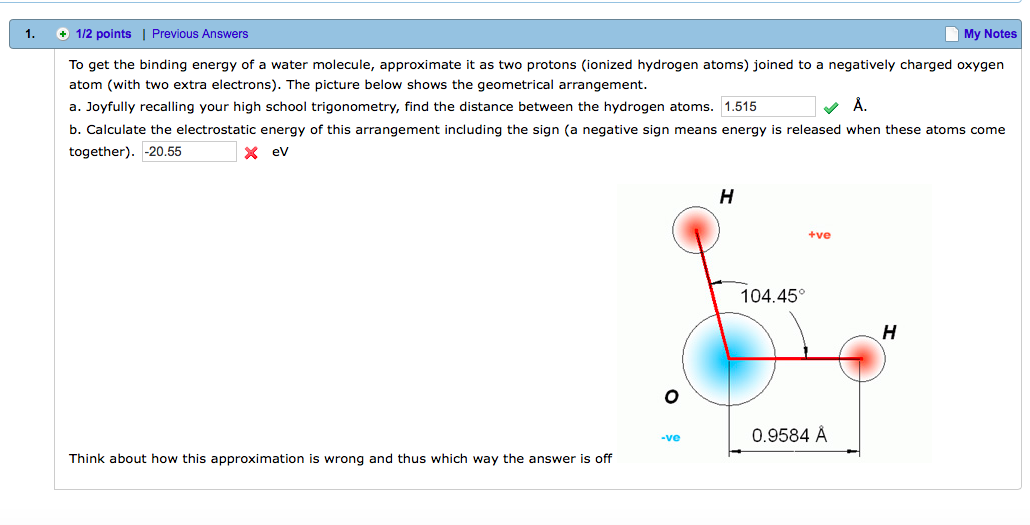
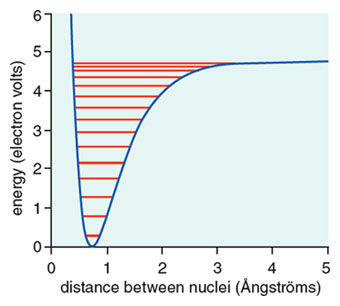
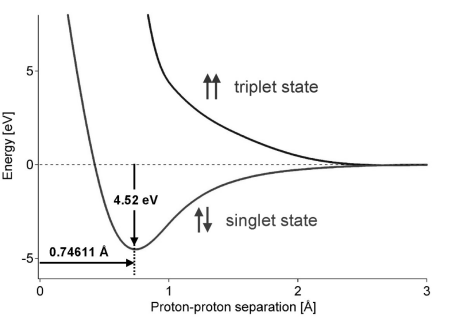
The binding energy of a hydrogen molecule is 4.75 eV. Energy required to dissociate 0.05% of hydrogen gas at NTP occupying volume 5.6 litres is:

Find the temperature at which the average kinetic energy of the molecule of hydrogen equals - YouTube
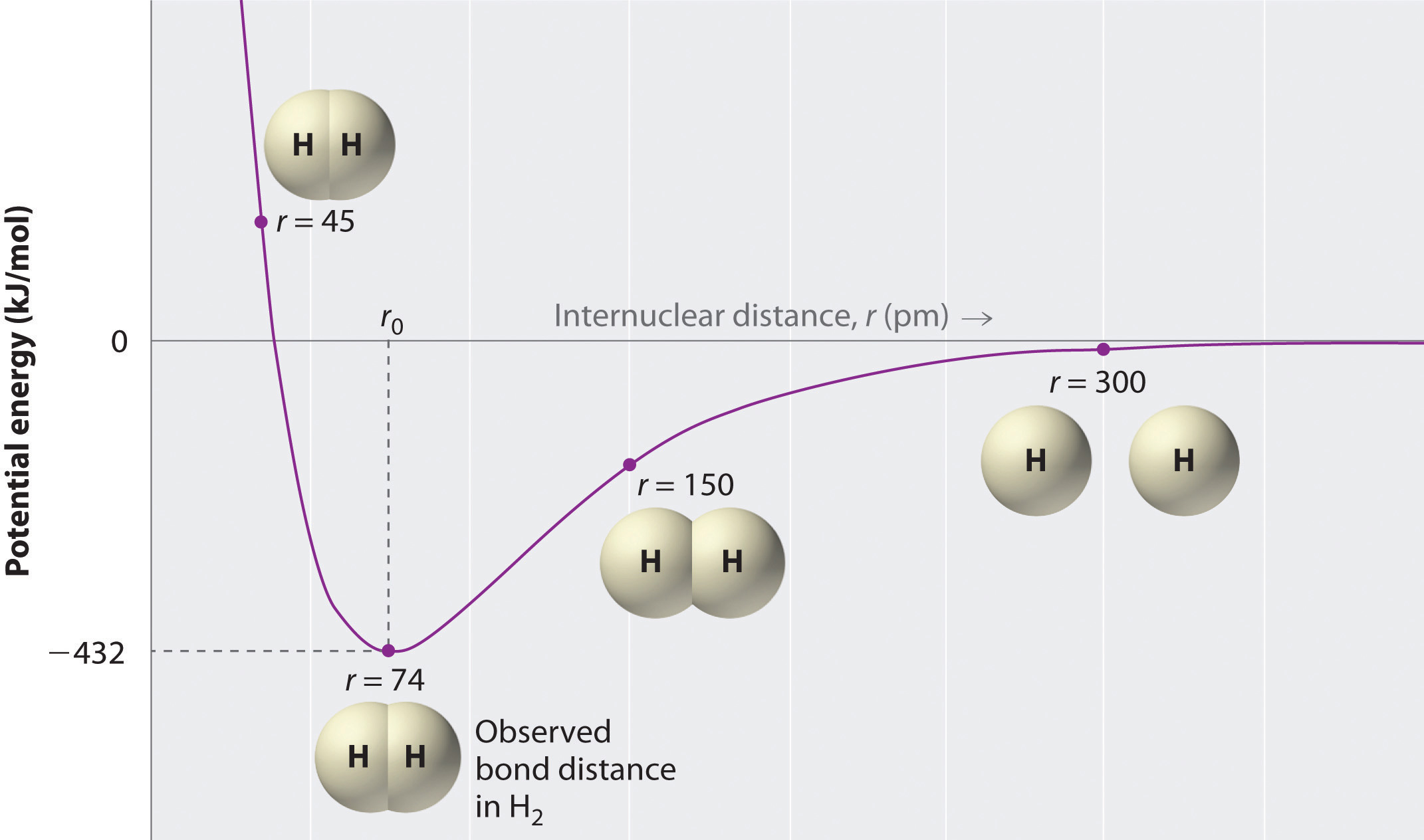
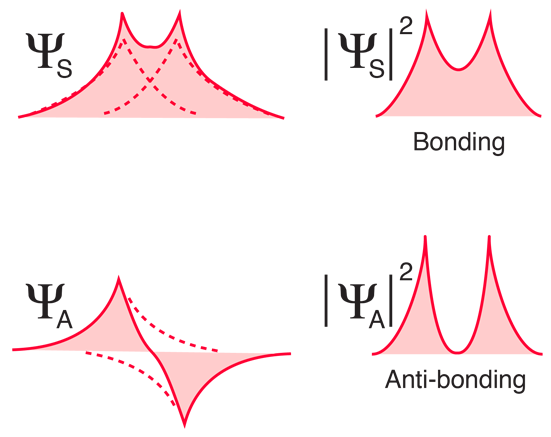
Two hydrogen atoms interact to form a hydrogen molecule. Classify the following statements that describe the stages of bond formation in a hydrogen molecule according to the predominant force existing between the

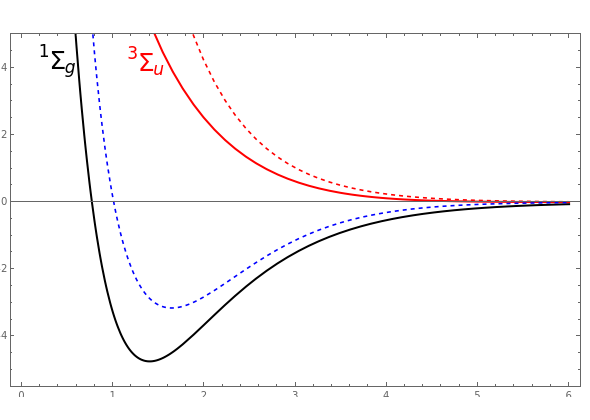
Colour on-line) Variational binding energy curves of the H2 molecule,... | Download Scientific Diagram
11: Binding energy curve of the H2 molecule calculated using the LDA... | Download Scientific Diagram



![PDF] The Ground State of the Hydrogen Molecule | Semantic Scholar PDF] The Ground State of the Hydrogen Molecule | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d4b2a0952df95b8c730ac486c0d2c767a138d22c/4-TableI-1.png)