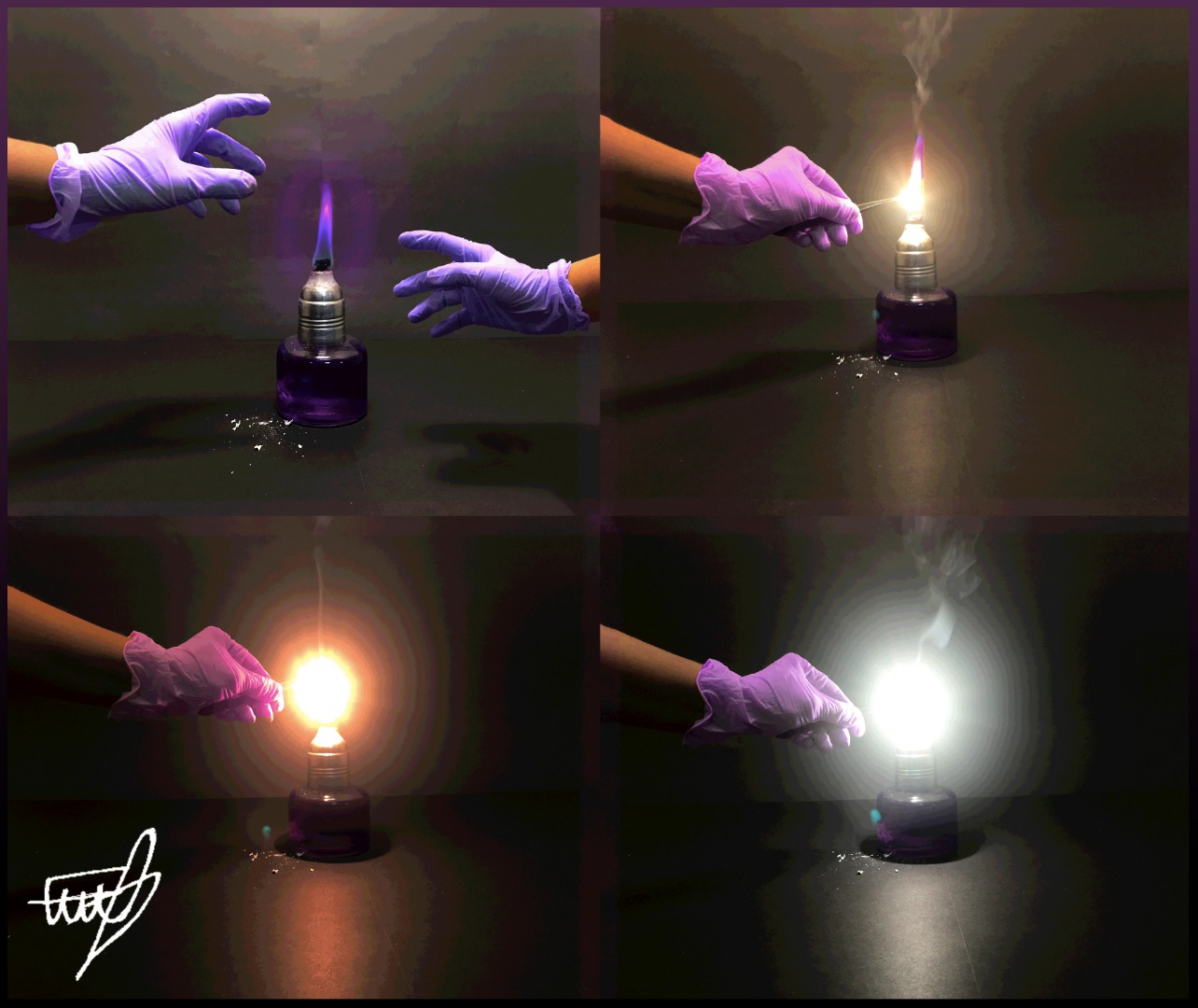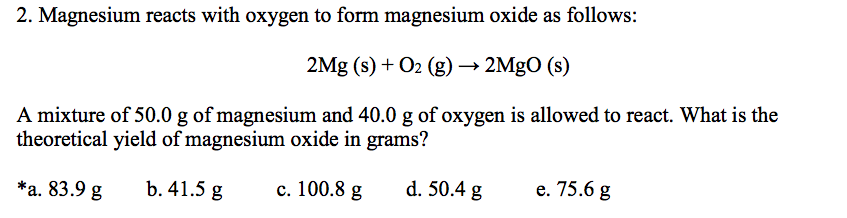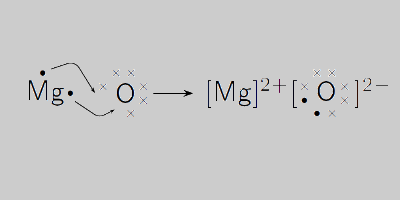
GCSE CHEMISTRY - The Reaction between Magnesium and Oxygen - Balanced Chemical Equation - Ionic - Bonding - Oxide - GCSE SCIENCE.

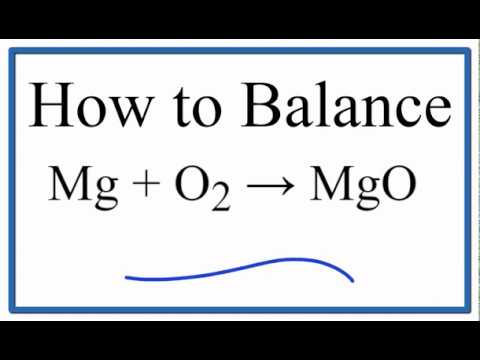
Write a balanced chemical equation for the following chemical reaction : Magnesium burns in oxygen - YouTube
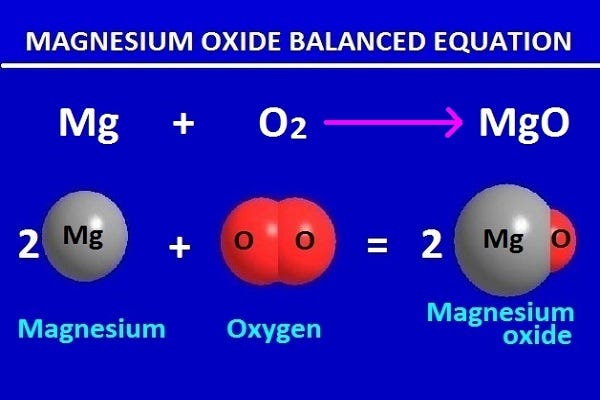
Magnesium oxide balanced equation in chemistry for class 9 | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

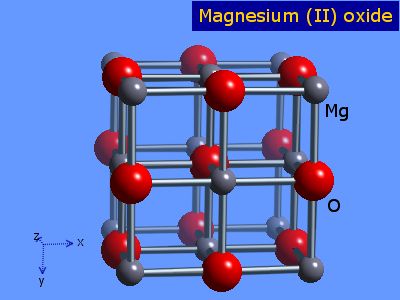
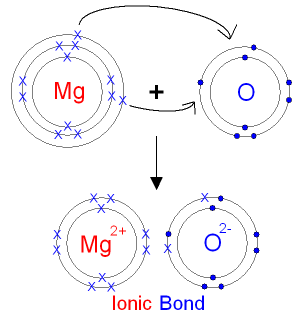
Diagram to show ionic bonding in magnesium oxide, Stock Vector, Vector And Low Budget Royalty Free Image. Pic. ESY-057091086 | agefotostock
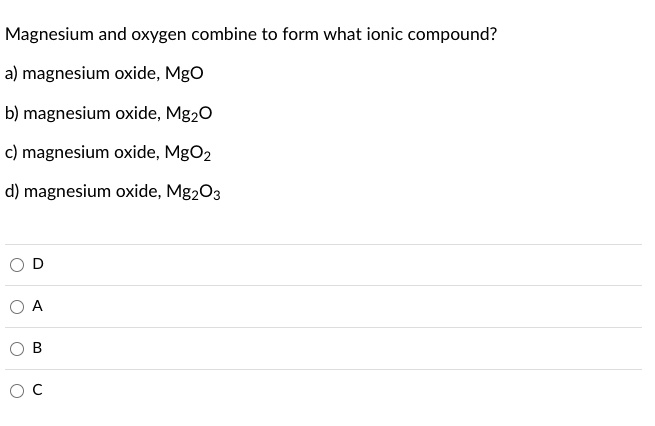
SOLVED: Magnesium and oxygen combine to form what ionic compound? a) magnesium oxide, Mgo b) magnesium oxide, MgzO magnesium oxide; MgOz magnesium oxide; MgzO3

Magnesium and oxygen combine in the ratio of `3 : 2` by mass to form magnesium oxide. What ma - YouTube

Question Video: Identifying the Oxidized Specie in the Reaction of Magnesium Oxide with Hydrogen | Nagwa